Transforming How Life Sciences Use Medical Imaging
Imaging studies are critical to the success of clinical trials for novel drugs and medical devices. Intelerad quickly transfers anonymized studies with strong storage and uptime processes, supporting you along the way with sponsor transparency and self-service.
No more shipping CDs, or ad hoc image transfer from multiple sending sites. Intelerad allows sending sites to upload images with just a weblink, customized portal, or CD upload tool. Now, it’s fast and simple to add new sending sites. With just a few clicks, you can support a time point, scale up time points, and add more patients – all while maintaining strong submission integrity.
Centrally managed and automated workflows enable studies to be routed to organizations, locations, groups, and users (like QA personnel and investigators) based on your approval processes and rules.
Anonymizing and de-identifying studies for clinical trials is often a manual process that is highly prone to error. Intelerad automatically removes DICOM tags locally, before the time point leaves the sending facility. This eliminates the risk of accidentally leaving any patient information tags in place; our software can even automatically remove identifiable information from the exam itself.
Don’t let poor imaging processes risk the integrity of your trial. Mailing in CDs or using on-premises archives heightens the risk of losing studies and lack of access due to unscheduled PACS downtime can slow down an entire trial. Intelerad’s SOC 2 compliant cloud storage and backup ensures that your imaging is always archived. Our software conforms to strong uptime SLAs and is accessible from anywhere.
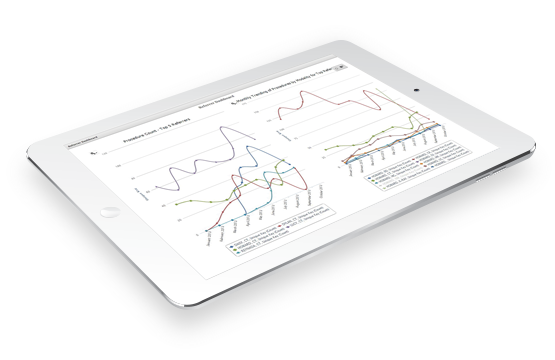
Intelerad makes it easy to provide exceptional service and transparency to trial sponsors. With a customized portal, sponsors will gain secure, self-service access to trial data, and can easily view and download studies. Intelerad offers built-in imaging reporting and analytics to provide you with detailed data like imaging volume, types of studies, time point trends and statistics, and much more.
Life Image’s Real World Imaging platform is the world’s largest evidence network specializing in ‘living’ data sets of novel imaging that are linkable to other clinical information. We are the only company on the market today to bring large, heterogenous, de-identified, and linked imaging data sets integrated to longitudinal clinical outcomes to support your real-world evidence programs.
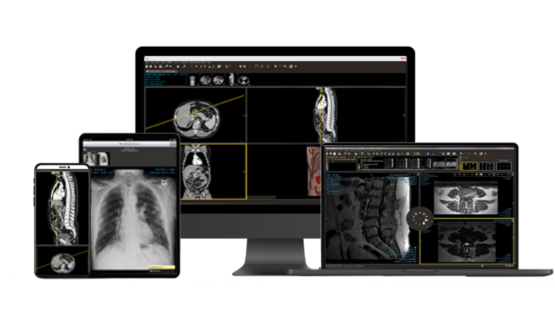

Give physicians easy access to patient orders, images, and reports, anywhere, anytime.

Customize a zero-footprint, web-based DICOM viewer, complete with advanced tooling like MIP, PET/CT fusion, MPR, and more.
Improve efficiency and standardization in mammography, patient tracking, and MQSA reporting through PenTrac’s comprehensive tracking and automation.
Drastically improve mammography reporting workflows by automating tasks and eliminating duplication.
Improve efficiency and standardization in mammography, patient tracking, and MQSA reporting through PenTrac’s comprehensive tracking and automation.
Enable patients to easily log in to a zero-footprint portal and gain direct access to exam history, images and reports.
Provide anytime, anywhere access to patients’ medical imaging with our zero-footprint, web-based patient portal.
Provide professional reporting and comprehensive fetal growth analysis throughout the gestational period.
Provide HIPAA-compliant, remote-reading access to the entire Digisonics OB/GYN image management and clinical reporting system via Citrix or VDI.
© 2021 Intelerad Medical Systems Incorporated. All rights reserved.